Hemin inhibits cardiac NaV channels
Hemin inhibits cadiac NaV channels
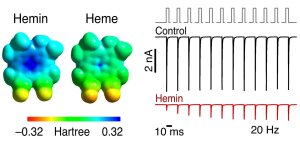
Abbildung: FSU BiophysikHere we show that the human cardiac voltage-gated sodium channel NaV1.5 is potently inhibited by extracellular Fe3+-protoporphyrin IX (hemin) (IC50 ≈ 80 nM), while heme, dimethylhemin, and protoporphyrin IX are ineffective. Hemin is selective for human NaV1.5 channels: hNaV1.2, hNaV1.4, hNaV1.7, and hNaV1.8 are insensitive to 1 µM hemin. Using domain chimeras of hNaV1.5 and rNaV1.2, domain II was identified as the critical determinant. Mutation N803G in the S3/S4 linker of domain II largely diminished the impact of hemin on the cardiac channel. This profile is reminiscent of the interaction of some peptide voltage-sensor toxins with NaV channels. The impact of hemin on NaV1.5 channels is reversely use dependent, compatible with an interaction of hemin and the voltage sensor of domain II. Extracellular hemin thus has potential to modulate the cardiac function.