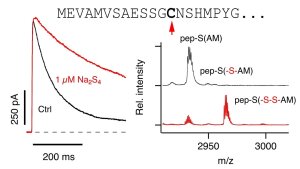
Sulfhydration eliminates potassium channel inactivation
N-terminal sequence of Kv1.4 (top); current traces of Kv1.4 before and after polysulfide application (left); MALDI spectra showing sulfhydration of C13 in the N-terminal inactivation peptide.
Illustration: FSU BiophysikHydrogen sulfide (H2S), which is enzymatically produced in various tissues, is increasingly recognized as an important signaling molecule in the cardiovascular and nervous systems. Here we show that H2S and its oxidized sulfur species polysulfides potently remove A-type potassium channel inactivation. Such channels determine action potential frequency in excitable cells. Polysulfides at nanomolar concentrations impair A-type channel inactivation by sulfhydrating cysteine residues in their N-terminal ball-and-chain domains. H2S and polysulfides therefore could be potent modulators of cellular electrical excitability by altering the availability of A-type channels and their kinetics of inactivation.