CO-releasing molecules standing offside?
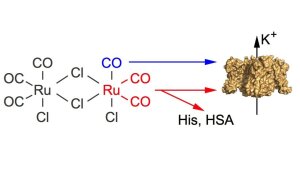
Dual effects of tricarbonyldichlororuthenium(II) dimer (CORM-2) on voltage-dependent potassium channels. Channel modification may result from binding of either carbon monoxide (blue) and/or of Ru(CO)2 fragments (red). The latter involves adduction to target histidines and, thus, can be quenched by excess free histidine or histidine-containing proteins such as human serum albumin.
Illustration: FSU BiophysikCarbon monoxide (CO), despite its toxicity, may serve as physiological messenger. To study the underlying molecular mechanism and for potential medicinal application, so-called CORMs (CO-releasing molecules), like tricarbonyldichlororuthenium(II) dimer (CORM-2), have been developed and frequently used. Using the patch-clamp technique, we show that for several K+ channels most of the functional impacts of CORM-2 are not mediated by CO but result from intermediate breakdown products: CORM-2 has the propensity of forming Ru(CO)2 adducts to histidine residues, as demonstrated using mass spectrometry. This off-site effect is completely abolished by excess free histidine or human serum albumin. Such adduct-formations may have been misinterpreted as CO gas effects in many previous and still ongoing studies, necessitating a re-assessment of derived interpretations thereof.