Fine-tuning of Kv channel inactivation by calcium
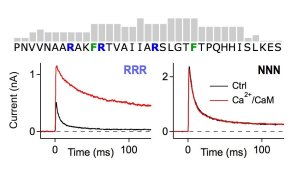
Score pattern for potential calmodulin binding to the N terminus of Kvβ1.1 (top) and current recordings of Kv1.1+Kvβ1.1 channels with and without Ca(2+)/CaM for wild-type Kvβ1.1 (left) and after replacement of three asparagine residues for arginine (right).
Illustration: FSU BiophysikSome auxiliary β subunits of voltage-gated K+ channels equip them with a rapid N-type inactivation mechanism, thus limiting K+ outward flow upon stimulation. Here we show that an elevation of the intracellular Ca2+ concentration removes Kvβ1.1-induced inactivation. This process is mediated by the Ca2+-binding protein calmodulin (CaM) binding to the “chain” element of the Kvβ1.1 N terminus, which normally operates as a “ball-and-chain” inactivation structure. By means of Kvβ1.1 subunits, voltage-gated K+ channels in neurons therefore are capable of functioning as negative feedback regulators to limit neuronal excitation and Ca2+ overload.