Multi-target ligands as potential drugs against neurodegenerative diseases
Neurodegenerative disorders such as Alzheimer's disease (AD) and Parkinson's disease (PD) pose major challenges to 21st century health systems. Because of their complex pathology, neurodegenerative disorders are unlikely to be mitigated by drugs that address only one clinically relevant target, and the requirement for the development of multi-functional drugs has been increasingly recognized. Several potential drug targets for the treatment of cognition and memory defects in AD patients or the extrapyramidal symptoms in PD patients have been identified. For example, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) degrade acetylcholine in the synaptic clefts of cholinergic neurons. The loss of cholinergic neurotransmission in the frontal cortex and hippocampus is a hallmark of AD, and AChE inhibitors that elevate acetylcholine levels, such as donepezil, have become first-line therapeutics to slow down AD progression. Glutamate mediates excitatory synaptic transmission in the brain, which is important for synaptic plasticity involved in long-term potentiation, memory, and learning. Pathophysiological conditions resulting in high extracellular concentrations of glutamate favor the excessive activation of N-methyl-d-aspartate receptors (NR), which leads to an overload of cells with calcium that eventually causes the loss of neurons. This process of NR-mediated, glutamate-induced excitotoxicity is effectively blocked by memantine, the only approved drug for the treatment of patients suffering from moderate to severe AD. Monoamine oxidases (MAO) catalyze the oxidative deamination of a variety of biogenic amines. Two types of MAOs that are encoded by different genes, show different tissue distribution, and display different substrate and inhibitor specificities. MAO-A inhibitors were the first antidepressant drugs developed, whereas MAO-B inhibitors with neuroprotective properties have been found useful for the therapeutic management of PD.
The concept of designed multiple ligands (DMLs), in which distinct pharmacophores of different drugs are combined in the same molecule, is particularly attractive for the treatment of disorders involving multiple pathophysiological processes. If bifunctional (heterobivalent) molecules are designed in which each pharmacophore binds to different clinically relevant targets, it may be assumed that this single-molecule combination therapy generates additive or synergistic effects that improve therapeutic efficacy. Another approach to creating DMLs would be the use of homobivalent molecules in which each half of the hybrid has identical binding properties, but at multiple targets that are not accessible for their monovalent analogs.
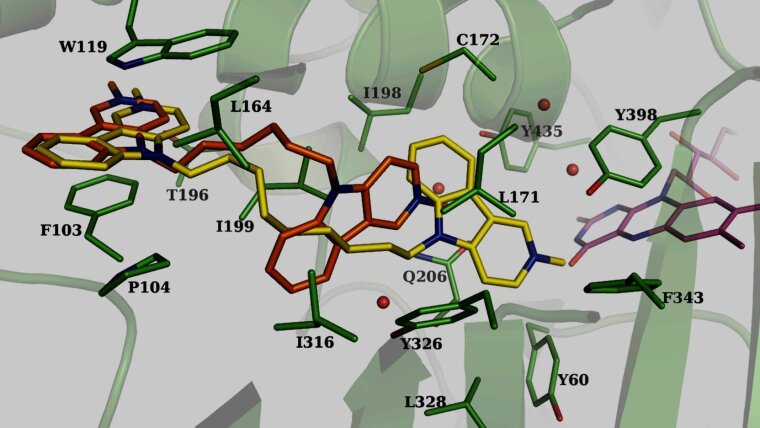
We evaluate homobivalent gamma-carbolines as potential DMLs that address three different targets relevant to the treatment of neurodegenerative disorders: cholinesterases, NR, and MAO.
Selected publications:
- S. Schwarthoff, N. Tischer, H. Sager, B. Schätz, M.M. Rohrbach, I. Raztsou, D. Robaa, F. Gaube, H.-D. Arndt, T. Winckler (2021). Evaluation of γ-carboline-phenothiazine conjugates as simultaneous NMDA receptor blockers and cholinesterase inhibitors. Bioorg. Med. Chem. 46, 116355 (PubMedExterner Link)
- C. Chen, D. Bönisch, R. Penzis, T. Winckler & G.K.E. Scriba (2018). Capillary electrophoresis-based enzyme assay for nicotinamide N-methyltransferase. Chromatographia 81, 1439-1444
- R. Otto, R. Penzis, F. Gaube, O. Adolph, K.J. Föhr, P. Warncke, D. Appenroth, C. Fleck, C. Enzensperger, J. Lehmann & T. Winckler (2015). Evaluation of homobivalent carbolines as designed multiple ligands for the treatment of neurodegenerative disorders. J. Med. Chem. 58, 6710-6715 (PubMedExterner Link)
- B. Tewes, B. Frehland, D. Schepmann, D. Robaa, T. Uengwetwanit, F. Gaube, T. Winckler, W. Sippl & B. Wünsch (2015). Enantiomerically pure 2-methyltetrahydro-3-benzazepin-1-ols selectively blocking GluN2B subunit containing N-methyl-D-aspartate receptors. J. Med. Chem. 58, 6293-6305 (PubMedExterner Link)
- R. Otto, R. Penzis, F. Gaube, T. Winckler, D. Appenroth, C. Fleck, C. Tränkle, J. Lehmann & C. Enzensperger (2014). Beta and gamma carboline derivatives as potential anti-Alzheimer agents: a comparison. Eur. J. Med. Chem. 87, 63-70 (PubMedExterner Link)
- Y. Rook, K.-U. Schmidtke, F. Gaube, D. Schepmann, B. Wünsch, J. Heilmann, J. Lehmann & T. Winckler (2010). Bivalent beta-carbolines as potential multi-target anti-Alzheimer agents. J. Med. Chem. 53, 3611-3617 (PubMedExterner Link)