Cell state transitions are per se alterations in the molecular identity – or in other words “phenotype” - of a cell to fulfill different functions. They occur naturally during embryonic development and are critical to generate the plethora of different cell types that constitute a creature, whereas in the adult organism they are mandatory to maintain body homeostasis. Converting a cell’s phenotype artificially in response to a specific stimulus is of highest interest in regenerative medicine. However, uncontrolled cell state transitions may also have disastrous consequences, when good cells turn bad and transform into a cancer cell.
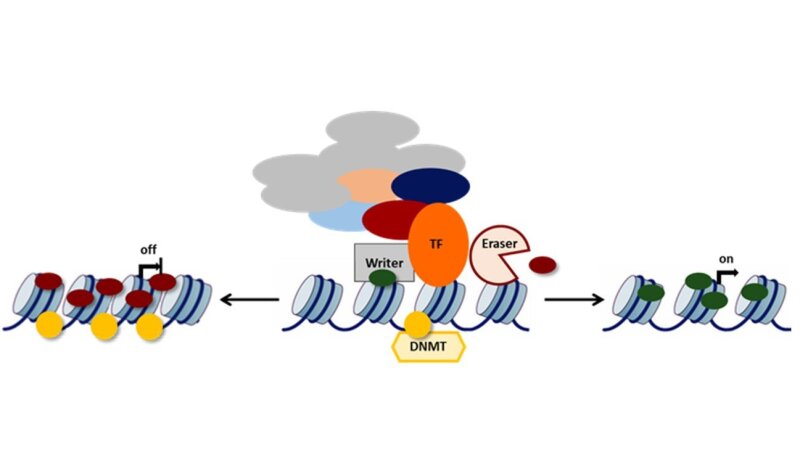
Since nearly all somatic cells and diploid germ cells share the same genome, an additional layer of information must exist to create phenotype diversity. This so-called epigenetic layer comprises the sum of biochemical information that is attached to the DNA itself (DNA methylation) or DNA packaging histones (methylation, acetylation, phosphorylation, etc. à posttranslational modifications (PTMs)), and non-coding RNAs for example, thereby influencing chromatin structure and eventually gene expression.
We are interested in how the dynamic interrelation between chromatin remodelers, epigenetic marks and transcription factors maintain a distinct cellular state or induce a transition.
We are studying genetically and epigenetically controlled cell state transitions during normal development, pathogenesis (e.g. inflammation) and aging in various biological systems.
Epigenetik
Image: Dept. of Biochemistry/FSU JenaTestis / Male Germ Cells
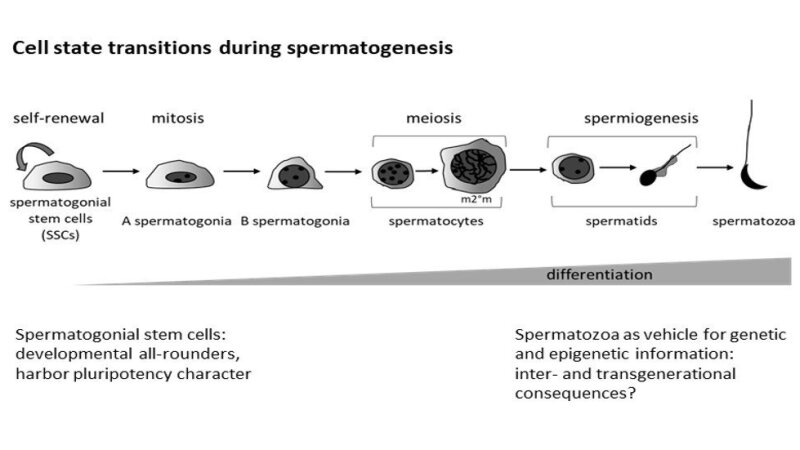
Though the importance of germ cells is undisputed – they guarantee the survival of a species – they often do not get enough attention in research. Still, germ cells are truly amazing cells: they are the only cells of the body that retain full developmental potential though they pass through different states of potency and, thus, through different molecular identities during a germ-line cycle. Moreover, they are the only cells of the body that transmit genetic and epigenetic information to the next generation. In our days, it becomes more and more evident that we are not necessarily stuck to our genes, but that leading a healthy lifestyle influences our phenotype. Thus, we should keep in mind that we may transfer (self-inflected) epigenetic errors to our children. However, these two characteristics makes germ cells most interesting for (a) studying general aspects of stem cell biology, (b) applications in regenerative medicine, and (c) uncovering the role of maternal and also paternal health in embryo development and offspring health.
Spermatogenese
Image: Dept. of Biochemistry/FSU JenaGeneral Research Questions:
- How does the epigenome drive germ cell state transitions?
- How do processes like aging influence the sperm epigenome and thus reproductive health and offspring phenotypes?
- How do transcription factors and epigenetic modifiers cooperate to enable proper spermatogenesis? What are the consequences of genetic errors and epimutations for spermatogenesis, fertility and offspring health?
Sandra Zemter, PhD candidate
Spermatogonial stem cells reside in seminiferous tubules of the testis and either self-renew to maintain the stem cell pool or they undergo several cell state transitions to eventually differentiate into mature spermatozoa. A complex interplay of transcription factors and epigenetic modifiers is responsible for this “decision” and can drive stem cell fate towards one direction. A strict balance between self-renewal and differentiations guarantees sperm production during a reproductive lifetime. We want to understand how transcription factors and epigenetic modifiers cooperate to drive spermatogonial stem cell fate during normal development and aging.
Peter Bohm, PhD candidate
The epigenome of germ cells is highly dynamic and comprises e.g. alterations in DNA methylation, histone PTMs and chromatin structure during germ cell state transitions. Alterations of the epigenome might not only affect proper spermatogenesis but also offspring health, as germ cells are able to transmit epigenetic information to following generations. Environmental influences and life style influence the sperm epigenome, and thus, a man’s behavior of today will shape the epigenome of tomorrow. We are interested in uncovering molecular mechanisms that drive epigenetic alterations and want to understand the impact of a dynamic epigenome for reproductive health and offspring phenotype.